Vstrip® COVID-19 Antigen Rapid Test





FEATURES
Method: Lateral Flow Immunoassay
Specimen: Posterior Nasopharynx Swab or Anterior Nasal Cavity Swab
Format: Dipstick / Cassette
Detection Time: 10 minutes
Storage Temperature: 15-30℃
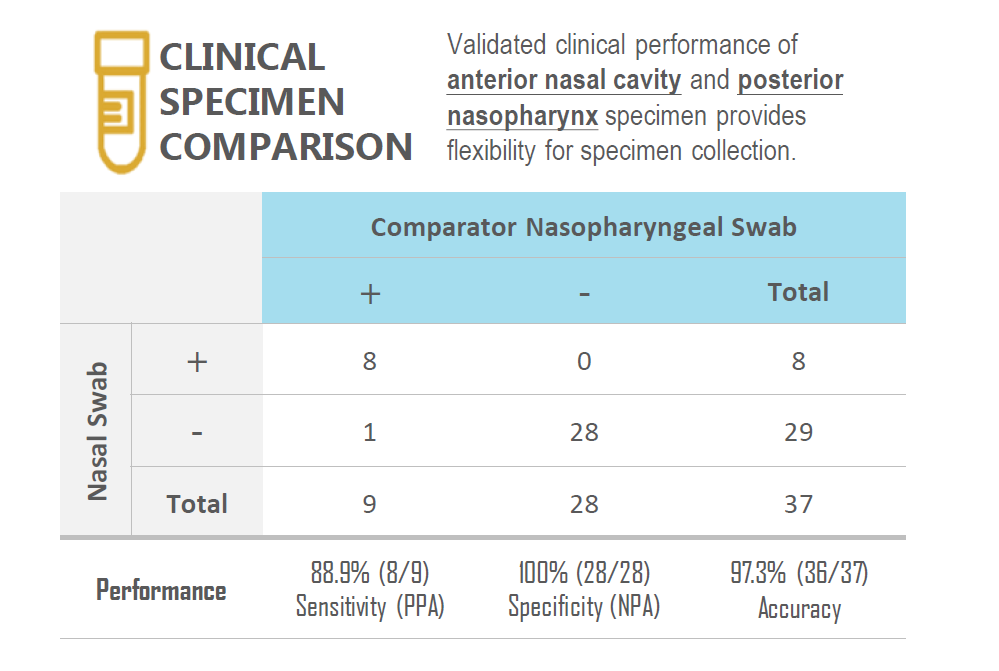
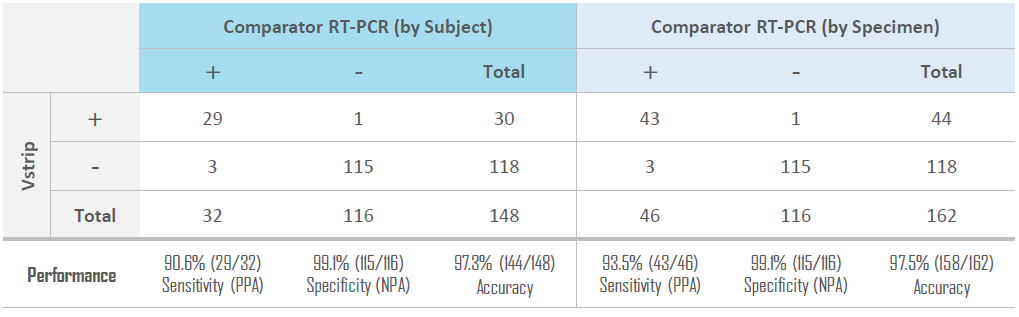
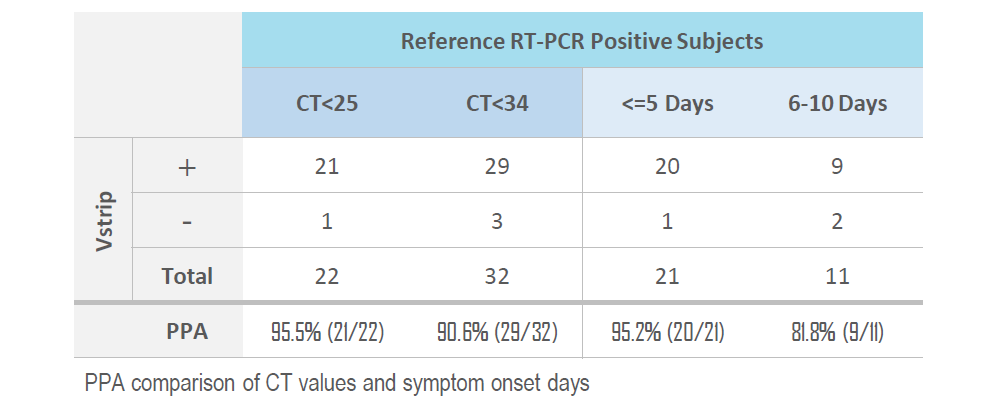
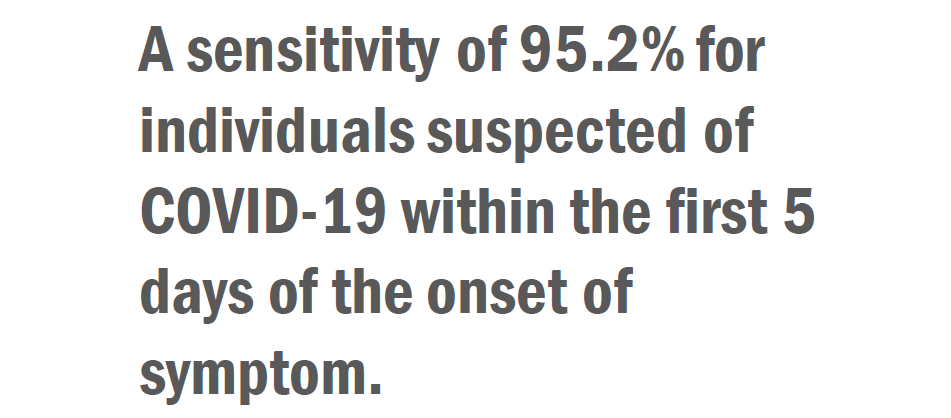
CLINICAL PERFORMANCE
The performance of the Vstrip COVID-19 Antigen Rapid Test for detection of SARS-CoV-2 was established with 148 individual symptomatic patients who were suspected of COVID-19
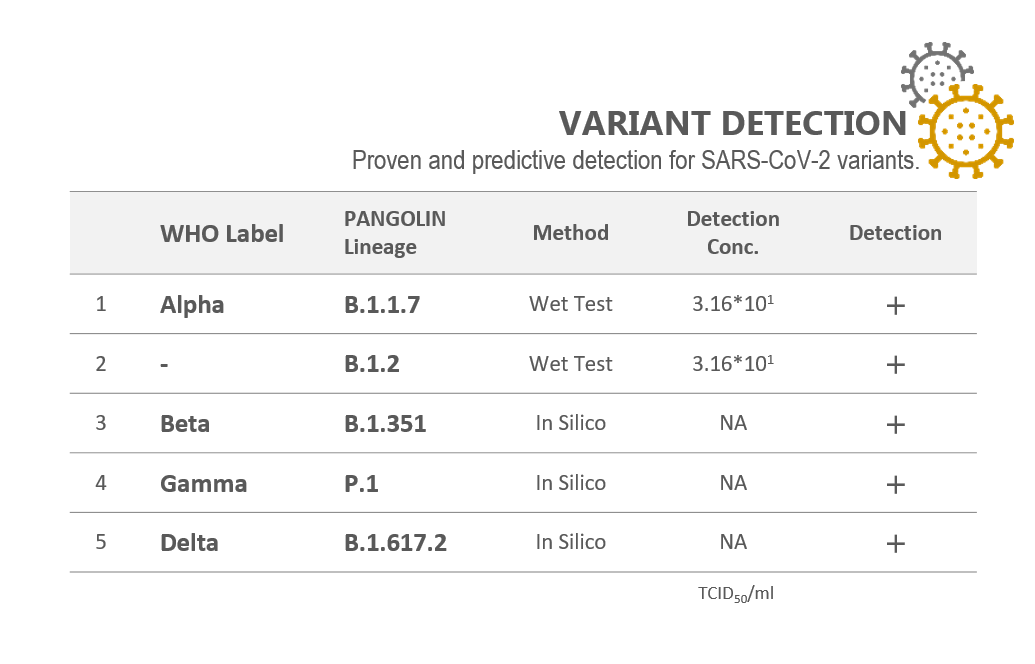
ANALYTICAL SENSITIVITY
Inactivated SARS-CoV-2 (USA-WA1/2020), gamma-Irradiated: 3.13x102 TICD50/ml
ANALYTICAL SPECIFICITY
The cross reactivity study was evaluated with a total of 12 bacteria strains and 15 viruses strains. None of these tested microorganisms gave a positive result
CERTIFICATION
ISO 13485:2016
TFDA_Case-Specific Approval of Disease Control for Manufacture No.1096813815
INDIA CDSCO Licence to Import Medical Device IMP/IVD/2021/000128
CE-IVD
SINGAPORE HSA Provisional Authorization
Philippines FDA Special Certification
